
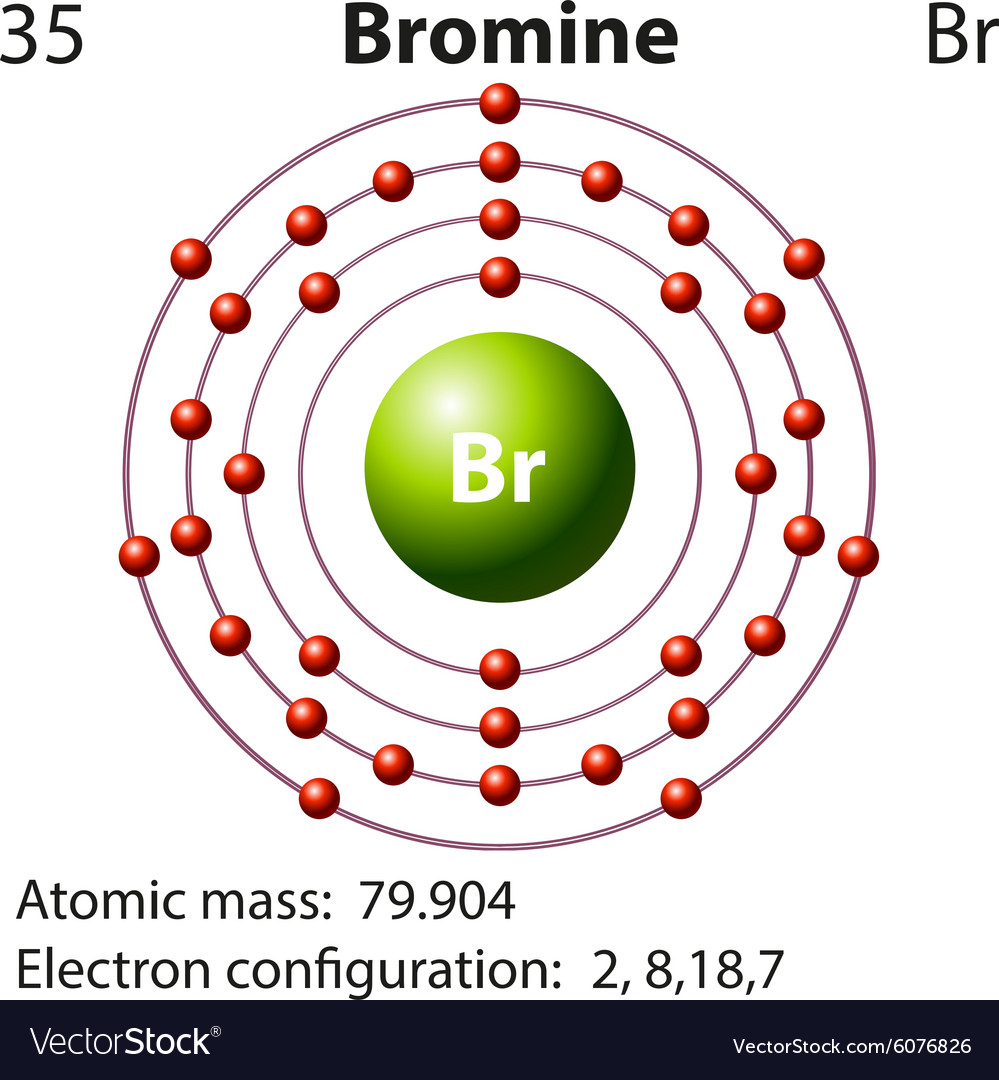
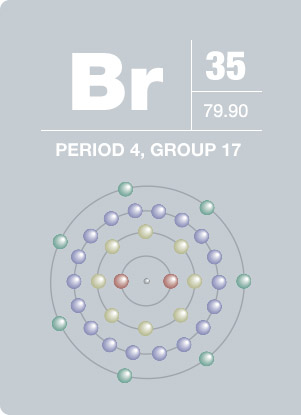
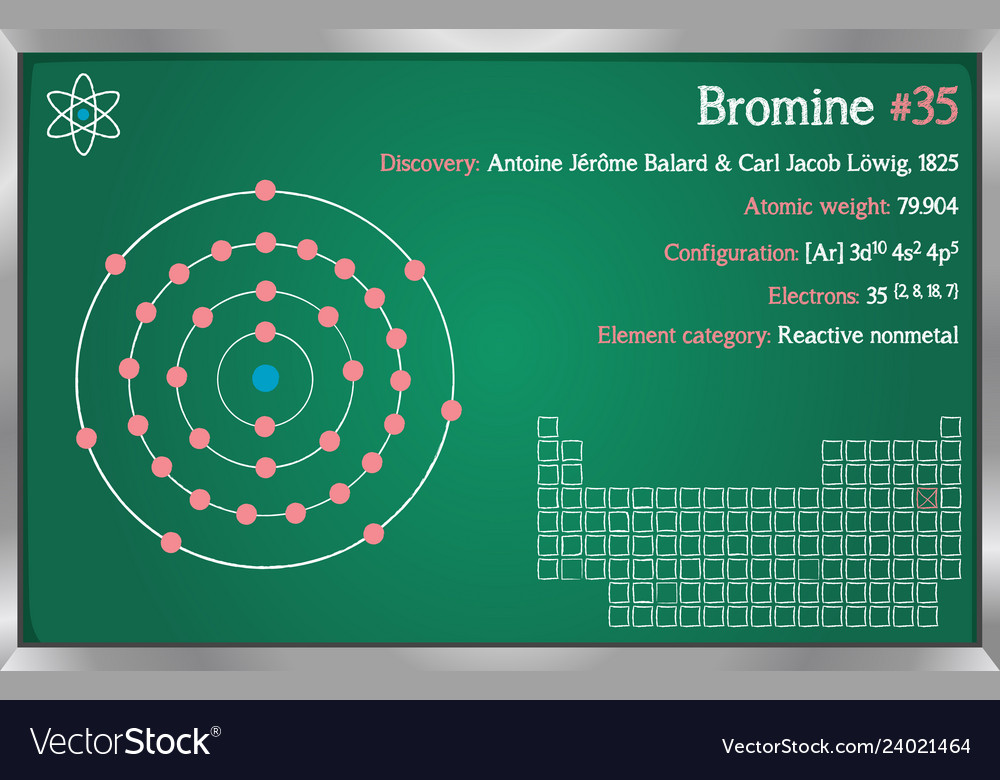
For Bromine the atomic number is 35. Sims 3 plus pets download mac. So number of protons = 35 In a neutral atom the number of protons = the number of electrons. 35 protons = 35 electrons. Gwyddion mac download. But Bromine anion with a charge of -1 has one extra electron so 35 +1 = 36 electrons. 36 electrons is the number of electrons in the stable inert gas Krypton. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br. Bromine is the third-lightest halogen, and is a fuming red-brown liquid at room temperature that. Bromine is a halogen - in Group XVII in the periodic table (along with fluorine, chlorine & iodine), with 7 electrons in its outer valence shell. As such, each bromine atom only needs one more to fill that outer shell and achieve a stable configuration (as the bromide ion Br-).
How many electrons are in the highest occupied energy level of bromine? How many electrons are in the highest occupies energy level of aluminum? Identify the element with an electron configuration of 1s^22s^22p^2. Identify the element with an electron configuration of 1s^22s^22p^63s^1. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electron configuration of ground state gaseous neutral bromine is Ar.3d 10.4s 2.4p 5 and the term symbol is 2 P 3/2.
How many electrons are there in #Br^-#?
1 Answer
Explanation:

Microsoft office publisher 2010 free download mac. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a
Below is the Lewis dot structure for a neutral bromine atom, which has seven valence electrons.
Below is the Lewis dot structure for a
Bromine Electrons
The diagram below shows how a bromine atom gains an electron from the element lithium in order to form the ionic compound LiBr.
Bromine Electron Structure
Related questions
