| The shell of the chicken egg is primarily calcium carbonate. |
A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol Ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. Calcium, a metallic element, is fifth in abundance in the earth's crust, of which it forms more than 3%. It is an essential constituent of leaves, bones, teeth, and shells. Never found in nature uncombined, it occurs abundantly as limestone, gypsum, and fluorite. Apatite is the fluorophosphate or chlorophosphate of calcium.
Its atomic number is 20. Its atomic mass is 40 u (approx). This discussion on What is the atomic no. And atomic mass of calcium? Is done on EduRev Study Group by Class 9 Students. Protons and Neutrons in Calcium. Calcium is a chemical element with atomic number 20 which means there are 20 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Calcium (Ca) is a silvery-white soft metal that has the atomic number 20 in the periodic table. It is an Alkaline Earth Metal and is located in Group 2 of the periodic table. It has the symbol Ca.
Calcium
| Atomic Number: | 20 | Atomic Radius: | 231 pm (Van der Waals) |
| Atomic Symbol: | Ca | Melting Point: | 842 °C |
| Atomic Weight: | 40.08 | Boiling Point: | 1484 °C |
| Electron Configuration: | [Ar]4s2 | Oxidation States: | +2, +1[3] (a strongly basic oxide) |
History

From the Latin word calx, lime. Though lime was prepared by the Romans in the first century under the name calx, the metal was not discovered until 1808. After learning that Berzelius and Pontin prepared calcium amalgam by electrolyzing lime in mercury, Davy was able to isolate the impure metal.
Sources
Calcium, a metallic element, is fifth in abundance in the earth's crust, of which it forms more than 3%. It is an essential constituent of leaves, bones, teeth, and shells. Never found in nature uncombined, it occurs abundantly as limestone, gypsum, and fluorite. Apatite is the fluorophosphate or chlorophosphate of calcium.
Properties
The metal has a silvery color, is rather hard, and is prepared by electrolysis of fused chloride and calcium fluoride (to lower the melting point).
Chemically it is one of the alkaline earth elements; it readily forms a white coating of nitride in air, reacts with water, burns with a yellow-red flame.
Uses
The metal is used as a reducing agent in preparing other metals such as thorium, uranium, zirconium, etc., and is used as a deoxidizer, desulfurizer, or decarburizer for various ferrous and nonferrous alloys. It is also used as an alloying agent for aluminum, beryllium, copper, lead, and magnesium alloys, and serves as a 'getter' for residual gases in vacuum tubes, etc.
Compounds
Its natural and prepared compounds are widely used. Quicklime (CaO), which is made by heating limestone that is changed into slaked lime by carefully adding water, is the great base of chemical refinery with countless uses.
When mixed with sand, it hardens mortar and plaster by taking up carbon dioxide from the air. Calcium from limestone is an important element in Portland cement.
Solubility of the carbonate in water containing carbon dioxide is high, which causes the formation of caves with stalactites and stalagmites and is responsible for hardness in water. Other important compounds are the carbide, chloride, cyanamide, hypochlorite, nitrate, and sulfide.
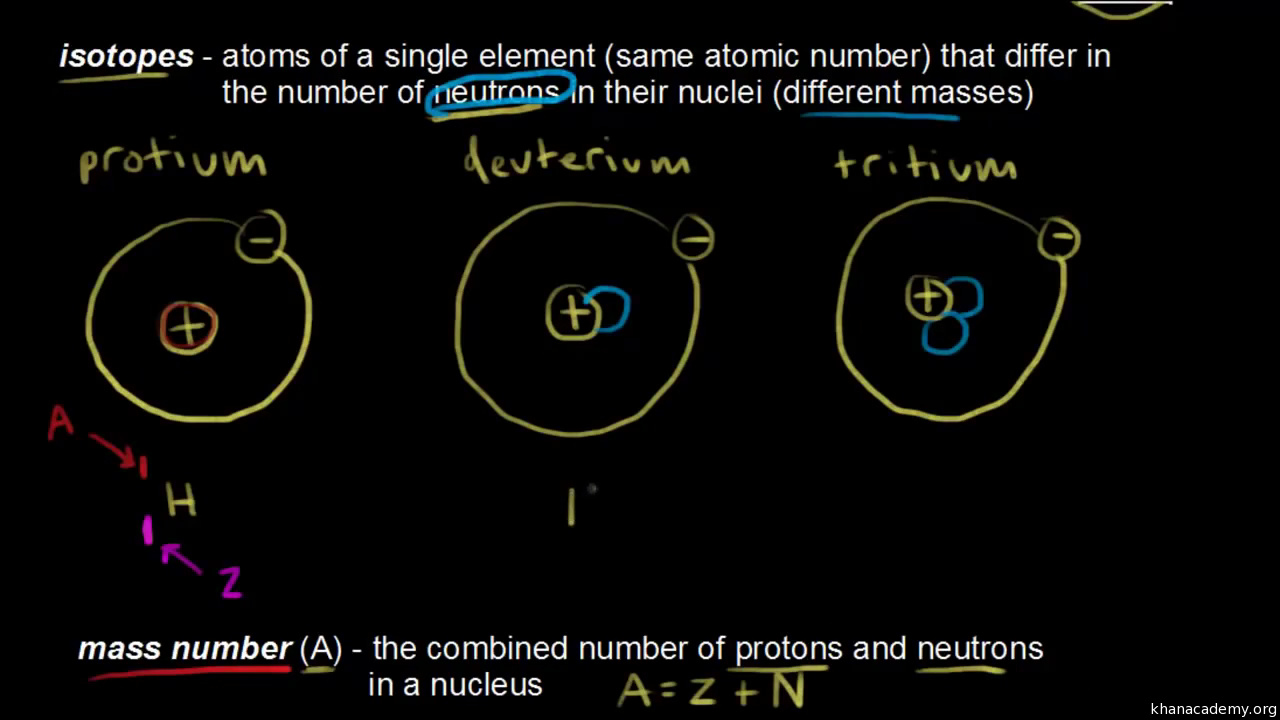
The element calcium has the atomic number of 20 and the mass number of 40, how many neutrons does it have?
2 Answers
Explanation:
There are few simple rules to follow.
(1) The atomic number is equal to the number of protons.
(2) In neutrally charged elements, the number of electrons is the same as the number of protons.
Otherwise, positive charge means that the element lost an electron and negative charge means it gained an electron.
(3) The atomic mass is equal to the sum of the number of protons and number of neutrons.
or
So if you say that
Atomic No Of Calcium
40 = 20 + number of neutrons
40 - 20 = number of neutrons
Therefore,
number of neutrons = 20
An atom of the calcium-40 isotope has
Explanation:
Isotopes are named for their mass numbers, for example, carbon-14. The mass number of an isotope is the sum of the number of protons and neutrons in the atomic nucleus. The atomic number is the number of protons.
Atomic No Of Calcium Fluoride
The calcium-40 isotope has a mass number of
Atomic No. Of Potassium
Related questions
